Markets
Possible applications & planning
The potential applications for our invention are broad, because there are many health conditions where weight gain can lead to health benefits.
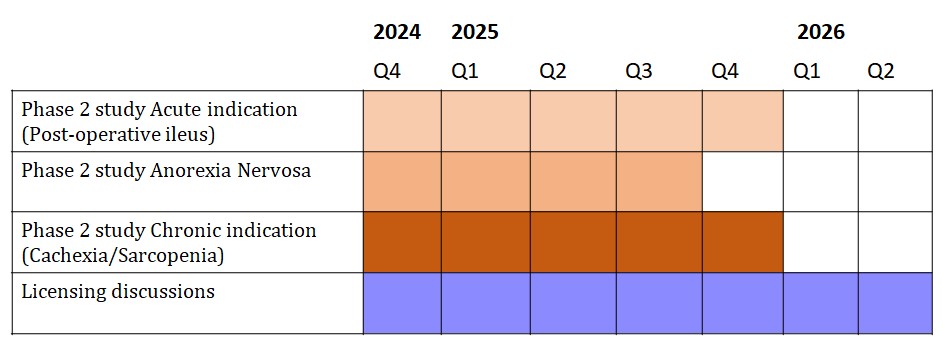
To prove that our invention works in patients we strive to conduct Phase 2 studies in the following indications:

Post-operative ileus:
Post-operative ileus (POI) occurs in up to 50% of patients who undergo major abdominal surgery (Kuruba et al., 2018). POI is characterized by the inability to tolerate a solid diet, delayed passage of flatus and formed stool, pain and abdominal distension, nausea, vomiting, and accumulation of gas or fluids in the bowel. The causes of POI are multi factorial and include surgical manipulation, inflammatory response, inhibitory neural reflexes, and the secretion of endogenous opioids within the gastrointestinal tract.
Every year over 20 million patients undergo open abdominal and laparoscopic surgeries in the major markets (US, EU5, China, Japan), and a half of these patients are expected to be eligible candidates for treatment.

Anorexia Nervosa:
Anorexia is a complex indication, with the main characteristic that patients refuse to eat. During therapy patients are coached to eat sufficient amounts. It is primarily a psychological problem but also pain and early satiety play a role. These patients could benefit from our drug, because it is expected that the food intake will be promoted. The question is however whether the patients will accept pharmacological treatment (to increase food intake).
The goal of this descriptive study is to answer the question whether there are Anorexia patients who accept benefit from the treatment with our drug.
Cachexia & Sarcopenia:
Cachexia is the loss of weight and muscle strength as a consequence of underlying disease (like cancer or COPD).
Sarcopenia is the loss of weight and muscle strength purely as a consequence of ageing. It is caused by changes in food intake and activity.
These are huge markets and we indicate a peak sales of > EUR 1 billion per year for Cachexia & Sarcopenia, if we can prove sufficient health benefits.
Planning: